Marine Scotland Blog
Marine Fund Scotland: North East marine projects net £5m
The Scottish Government recently opened its fourth year of the £14m Marine Fund Scotland for 2024-25.
Applications are welcome until Thursday 9 May 2024 from a wide range of eligible individuals, businesses, organisations and communities to deliver projects which contribute to an innovative and sustainable marine economy.
More than a third (33) of the 91 projects awarded funding in 2023-24 were businesses in the North East of Scotland, totalling £4.8m in grants. On an individual basis, these ranged from around £6k to £1.16m.
Fraserburgh Harbour benefitted from grants to make harbour improvements, including around £1.16m for North breakwater structural repairs and more than £87k on a decarbonising clean up using an all-electric boat and marine litter prevention campaign.
Pamela Neri, Harbour Development Manager for Fraserburgh Harbour said:
“We are greatly appreciative of the funding assistance from the Scottish Government, which has provided current infrastructure and future environmental enhancements to the harbour.
“Together with the community we have been working with local schools to educate on the challenge of marine litter, bringing the issue to the wider public and addressing how we tackle it together.”
Robert Gordon University secured around £360k for a research and innovation project to develop biotoxin testing for shellfish.
Robert Gordon University (RGU) Applied Microbiology Professor, Christine Edwards said:
“Making sure our shellfish is safe to eat is a legal responsibility for all food business in Europe.
“Unlike some other harmful bacteria and viruses which can cause food poisoning, biotoxins are largely resistant to heat so will not be removed through cooking. That’s why testing for biotoxins in Scottish shellfish, enjoyed at home and across the globe, is paramount to make sure product containing unsafe levels is not placed on the market.
“CyanoSol research group which includes colleagues from RGU, Scottish Biologics Facility at University of Aberdeen and Lateral DX Ltd continue to work closely with industry partners to develop robust solutions to new field tests for diarrhetic shellfish poison toxins.”
In Aberdeenshire, Trinity Seafoods (formerly Peterhead Whitefish Processors) received more than £360k for the purchase and installation of a white fish processing line. This automated facility provides a solution to the growing problem of manually handling smaller sizes of whitefish species, which is a time consuming and costly process. This will reduce waste and increase utilisation of this important blue food resource.
Richard Duthie, Trinity Seafoods Director, said:
“It is great to see the Scottish seafood sector collaborating to create this new venture that will benefit fishermen and processers, and ensure best use is made out of sustainably caught whitefish.
“Not only will the new company and equipment offer direct benefits for the fishing and processing sectors, it will boost many support businesses in the supply chain.”
As well as seafood processing, marine research and innovation, and harbour improvements, other projects supported in the North East last year included fishing vessels, young fishers, commercial fishing and aquaculture. North East locations range from Aberdeenshire and Moray to Aberdeen and Angus.
Full list of the beneficiaries for 2023-24.
The post Marine Fund Scotland: North East marine projects net £5m appeared first on Marine.
Faroe fisheries agreement delivers for Scotland’s fishing industry
Bilateral fisheries negotiations with the Faroe Islands, one of Scotland’s closest fishing neighbours, have now concluded for 2024. These agreements set out exchanges of fishing opportunities of quotas and access.
The UK/Faroe agreement sets out quota exchanges which will allow UK vessels to fish key species in Faroese waters. This includes quotas for cod, haddock, and saithe at similar levels to the 2023 agreement in exchange for stocks including Greenland halibut, North Sea haddock and Western blue ling.
Cabinet Secretary for Rural Affairs, Land Reform and Islands Mairi Gougeon said:
I am pleased to confirm that an agreement was reached between the UK and the Faroe Islands on 1 March. This was the final in a suite of negotiations which have cumulatively provided over £600 million of fishing opportunities to Scottish fishers in 2024.
The Scottish Government has been fully involved in the negotiations, with these talks highlighted as a key priority by many in our industry. This deal sees exchanges of quota and access for Scottish vessels into Faroese waters for species such as cod, haddock and saithe in 2024.
The outcomes will provide additional opportunities and flexibility, enabling our larger whitefish vessels to divert their effort into Faroese waters, in turn putting less pressure on stocks in Scottish waters. This deal also provides a platform to continue to build on our already strong relationship with the Faroe Islands as we seek to manage our fish stocks sustainably.
Background
The UK signed Framework Agreements on Fisheries in 2020 with the Faroe Islands. Bilateral exchanges of opportunities were also agreed in 2022 and 2023.
Through the UK/Faroe agreement, Scottish vessels will be able to fish key whitefish stocks including cod, haddock, and saithe in Faroese waters. The UK has exchanged out quotas including Greenland halibut, haddock, blue ling, tusk and ling.
The UK/Faroe Agreed Record is published on the Scottish Government website.
The post Faroe fisheries agreement delivers for Scotland’s fishing industry appeared first on Marine.
Sixth annual ScotMER symposium draws record attendees
The ScotMER programme recently held its sixth annual symposium attracting over 1,000 registered attendees from 30 different countries.
The symposium took place online over three days and showcased the breadth of active research currently being undertaken by the programme. Attendees heard updates from each of the seven ScotMER receptor groups (described below), as well as the ECOWind and OWEC Research Programmes.
For anybody that missed it, all the talks are now available on the Marine Directorate YouTube Channel and more information about the ScotMER programme itself is below.
ScotMER
The Scottish Marine Energy Research Programme (ScotMER) is a Scottish Government initiative that identifies and addresses key evidence needs to help inform licensing, consenting and planning decisions concerning offshore renewable developments.
The Scottish Government has committed to investing up to £3.2 million per year until 2026/27 into research that will be delivered through the ScotMER programme, to improve the scientific evidence base that is key to delivering ScotWind.
ScotMER supports the Scottish Government’s commitment to Net Zero by 2045 and provides evidence for the Sectoral Marine Plan for Offshore Wind Energy and the National Marine Plan. Evidence produced by ScotMER helps to deliver towards:
Our Blue Economy Vision for Scotland
Scottish Government’s Climate Change Plan 2018-2032
Draft Scottish Biodiversity strategy to 2045: tackling the nature emergency
Draft Energy Strategy and Just Transition Plan
ScotMER develops the evidence base for these plans through three areas:
Receptor Groups
The ScotMER programme has seven receptor groups shown in the image below. Each receptor has a dedicated group of technical experts who specialise in a specific part of the marine ecosystem. Members consist of a range of external and internal stakeholders from across government (marine science, planning and licensing), statutory nature conservation bodies (e.g. NatureScot), academia, industry (developers via Scottish Renewables developer representatives, and representatives from the fishing industry), as well as environmental non-governmental organisations (eNGOs).
The ScotMER receptor groups are listed below:
Research
Research ideas proposed by the receptor groups are reviewed internally by the Scottish Government, then developed into project ideas. These are prioritised for progression based on current evidence needs by the projects board, a collection of key internal and external stakeholders, before presenting to ministers for approval.
In the last year the ScotMER programme has published seven project outputs on the Scottish Government website. The programme currently has fourteen active research projects, and a series of new projects in planning.
Communication
The ScotMER Programme actively communicates the project outputs to key decision makers across Scottish Government (marine licensing, consenting, planning and policy colleagues), as well as external stakeholders such as NatureScot, industry (developers and fishing), the academic community, and environmental non-governmental organisations.
Along with publishing project outputs, the programme has presented at conferences such as All Energy and the Marine Alliance for Science and Technology for Scotland (MASTS) Annual Science Meeting. We also engage with external stakeholders through broader engagement and collaboration with other research programmes such as the Offshore Renewable Joint Industry Programme (ORJIP). This ensures the research interests of ScotMER are aligned with other work ongoing in the marine renewable energy sector and avoids duplication of effort.
The post Sixth annual ScotMER symposium draws record attendees appeared first on Marine.
Marine Fund Scotland case study: Seafood in Schools
A part of the 2022-23 Marine Fund Scotland, Scottish Government awarded £40k to Seafood Scotland’s ‘Seafood in Schools’ scheme. This educational initiative teaches school pupils about the health benefits of seafood, as well as providing an insight into the range of careers available across the marine supply chain.
Seafood Scotland Chief Executive Officer, Donna Fordyce shares more:
“Engaging with school children and their influencers is vital for our sector. It helps us inform them not only how tasty our seafood is, but also of the nutritional value of eating seafood. That it’s versatile and economical to cook, and how vital and varied this sector is in terms of the species you can find around Scotland.
“We realised the importance of this, not only to ensure young people are eating seafood, but at a time when we’re seeing huge skills shortages across the industry – it’s imperative that we educate young people on the opportunities available to them within the seafood sector.
“Following a successful pilot scheme with primary schools in the northeast, Marine Fund Scotland provided funding to support this initiative to roll out the workshops across central Scotland.
“The initial trial programme had consisted of five workshops in Peterhead and Fraserburgh primary schools, tapping into an area that’s at the heart of our seafood sector. The success of these and the funding allowed for a further series of 31 workshops across the central belt, reaching primary 4, 5 and 6 pupils.
“The workshops were dynamic and fun including a short presentation on seafood – what species there are, the nutritional benefits, how sustainable the sector is and why it’s important to our local and national economies. This was followed by interactive games and a cookery workshop where the children got to sample salmon, whitefish, and mackerel dishes. This proved a huge hit with the children and teachers – some of them tasting these fish for the first time.
“As a takeaway to the workshops, all pupils were given recipe cards on the dishes they tried, along with some tins of mackerel, rice or noodles and stationary items. This meant the children could recreate the dishes at home and talk to their parents about what they’d learnt that day.
“We are very grateful to the Scottish seafood sector for supporting this initiative and supplying free products for us to use in schools.
“The project surpassed expectations, with over 800 pupils taking part (our target had been 500), 16 schools hosting us, four more than our target of 12, and following a full evaluation amongst teachers and pupils, the feedback was 100% positive.
The results showed 58% of pupils would want to eat more seafood, 83% tried seafood they hadn’t tasted before and 84% said they learnt something new about seafood.
“As the next stage of the initiative, we’re looking to target secondary school children, specifically Senior 2 pupils who are about to choose their subjects to take to exam level. We’re currently setting up pilot workshops in Fraserburgh and Peterhead with similar activity to the primary workshops, but with more of a focus on the vocational opportunities there are across the sector. As part of our informative session, we’re talking through a ‘sea to plate’ journey across the seafood sector, highlighting the breadth of jobs needed to bring fish to the dinner plate.
“The hope is that following these workshops, more funding is available to expand the secondary schools’ workshops into more areas to help us encourage school leavers to find work in the sector and continue to build on our vital industry.”
The post Marine Fund Scotland case study: Seafood in Schools appeared first on Marine.
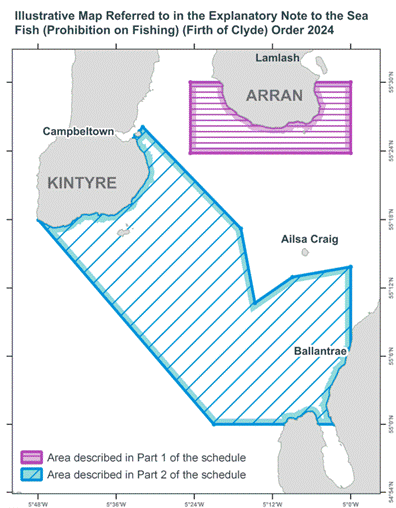
Seasonal closure to protect spawning cod in long-term interests of fishers and marine environment
An area of the Firth of Clyde will again be closed to most types of fishing activity in spring 2024 and 2025 to protect spawning cod.
The closure has been in place each year since 2002 during the cod spawning season. While there has been variation, the closures in 2024 and 2025 will be on the same basis as in 2022 and 2023, prohibiting most types of fishing activity for eleven weeks from 14 February to 30 April.
During spawning, cod are vulnerable to disruption. Cod mate by lekking, a behaviour also found in birds such as black grouse and capercaillie. Male cod become territorial and take possession of an area of the seabed, defending it from other males. They use muscles around their swim bladders to make grunting and rumbling sounds to attract females. When focussed on mating, cod are less likely to try and avoid fishing gear so are particularly vulnerable to being caught. If disturbed, cod are unlikely to return and may not mate at all that year, with research suggesting that any activity within 10m of the seabed could disrupt spawning activity.
The closure takes account of the cod’s preference to spawn on gravel and coarse sand, with these areas closed. Surrounding areas, where the seabed is softer sand and mud and cod are less likely to spawn, remain open to fishing.
We consulted on renewing the management measures last year with a majority of respondents supporting the closure.
The eleven-week closure will have short-term impact on local fishers. Fishing for nephrops in the closure area will be prohibited due to the risk of inadvertently catching cod (by-catch), or otherwise disturbing cod spawning sites with fishing gear. However, the closure is part of a range of fisheries management measures which aim to replenish cod stocks in the medium to longer term, creating a more sustainable fishery benefitting our marine eco-system and fishing industry in the West of Scotland.
We base fisheries management decisions on the best available science. Recent ICES advice on northern shelf cod (the wider stock which includes cod found spawning in the Firth of Clyde) is much more positive than it once was, showing a stock in recovery. However, it is still appropriate for us to take a precautionary approach with robust management measures to protect spawning and juvenile fish in our inshore waters.
We will be enhancing data collection and monitoring in the Clyde during the closure to help inform future management decisions. We are also committed to working with our Coastal States partners Norway and the EU to review the management measures in place for the wider northern shelf cod stock.
More information
The closure for 2024 and 2025 is implemented by a Scottish Statutory Instrument (SSI) laid in the Scottish Parliament on 11 January 2024. The closure is in effect from 14 February to 30 April in 2024 and 2025.
The post Seasonal closure to protect spawning cod in long-term interests of fishers and marine environment appeared first on Marine.
Protecting Wild Salmon
Net Zero Secretary Màiri McAllan visited Kelso for the opening of the 2024 salmon fishing season on the Tweed and to launch the Sustainable Rivers Audit.
She joined the Duchess of Sutherland, the River Tweed Commission, River Tweed Foundation, The Tweed Forum, fishers and community members to mark the formal opening of the river before launching the Audit, which will help improve understanding of where management action needs to be taken to benefit wild salmon.
Màiri McAllan said: “I was very pleased to be in the Borders to take part in the opening of the 2024 season and to launch the Sustainable Rivers Audit.
“Atlantic salmon are one of our most iconic species and Scottish Government is committed to working with our partners, both domestic and international, to protect and restore populations.
“Free access to cold, clean water is essential for wild salmon. A year ago we published our Wild Salmon Strategy Implementation Plan which sets out over sixty actions to tackle the wide range of pressures on wild salmon, including planting riverside trees to protect rivers from rising temperatures and restoring natural river flows by removing weirs that are no longer in use.
“The work that will be carried out as part of the Sustainable Rivers Audit will therefore be invaluable to us all in better understanding and improving the catchment and we are grateful to the Tweed Commission and Foundation as critical partners in our efforts.
“I look forward to seeing the results of the audit and I wish everyone a successful 2024 season.”
Jamie Stewart, Chief Executive of the River Tweed Commission said: “We were really pleased to be able to host the Cabinet Secretary. Her knowledge and contribution to the debate was well received, as was her commitment to visit again to discuss in detail our ongoing relationship with other stakeholders to ensure we have the best opportunity to have cold clean water to support those returning salmon and the nurseries for the future.
“We look forward to working with the Cabinet Secretary and her parliamentary colleagues as we work towards a partnership to mitigate the decline in the iconic Atlantic salmon.”
This follows the announcement this week of the award of grants worth £14 million from the Marine Fund Scotland 2023-24, including several aimed at protecting and conserving wild salmon populations.
Fisheries Management Scotland, the representative body for District Salmon Fishery Boards and Rivers/Fisheries Trusts, has received a combined £750,000 for four projects. This will include the management of invasive non-native pink salmon in Scotland as well as the purchase of equipment to support monitoring.
Dr Alan Wells, CEO of Fisheries Management Scotland said:
“Fisheries Management Scotland is extremely grateful for this important support from Marine Fund Scotland, which recognises that Scotland’s wild Atlantic salmon are a conservation priority. Our members are working tirelessly to protect, conserve and restore Scotland’s wild salmon populations and this vital funding will support these crucial efforts.
“This funding will directly support actions identified in Scotland’s Wild Salmon Strategy. It will support efforts to understand the abundance of young salmon in our rivers, identify and quantify pressures, and highlight the management actions required to address them. It will also support practical action to manage pressures faced by Scotland’s wild salmon, including predation and invasive, non-native pink salmon.”
BackgroundWild Salmon Strategy Implementation Plan
The post Protecting Wild Salmon appeared first on Marine.
Sandeel fishing to be banned in Scottish waters
Fishing for sandeel is to be banned in Scottish waters ahead of the 2024 fishery season, subject to Parliamentary approval.
Sandeel support the long term sustainability and resilience of the marine ecosystem and are an important food source for many species, including marine mammals, seabirds and predatory fish.
Commercial fishing for sandeel is currently carried out entirely by European vessels. In recognition of the importance of the species to marine biodiversity, no fishing quota has been allocated to UK vessels since 2021.
Cabinet Secretary for Rural Affairs, Land Reform and Islands Mairi Gougeon said:
“Sandeel are a vital part of our marine ecosystem and a critical component of the food chain in the North Atlantic.
“It is critical that we manage our marine environment in a such a way as to ensure its sustainable use, protecting biodiversity and ensuring healthy functioning ecosystems.
“Prohibiting all vessels from fishing for sandeel in Scottish waters will help provide long term sustainability and benefits not just for sandeel but also for seabirds, marine mammals and other fish species.
“This decision reflects overwhelming support for our proposals, the scientific evidence base and our longstanding position not to support fishing for sandeel as set out in Scotland’s Future Fisheries Management Strategy.”
The Scottish Government consulted on proposals to close fishing for sandeel in Scottish waters in 2023, with 97% of respondents indicating support for the preferred option.
The UK Government has today also indicated its intention to close Area 4 of the North Sea in English waters for sandeel fishing.
Background
The SEA FISHING (PROHIBITION ON FISHING FOR SANDEEL) ORDER 2024 will be laid before Parliament on 5 February.
The Order is subject to a negative procedure and if approved and made, will come into force on 26 March 2024
Future Fisheries Management Strategy
The post Sandeel fishing to be banned in Scottish waters appeared first on Marine.
Bilateral Norway agreement brings additional fishing opportunities
Consultations with Norway on exchanges of fishing opportunities with the UK in 2024, including quotas and access, concluded with the signing of an Agreed Record on 14 December. Fishing activity has now commenced in both UK and Norwegian waters under the terms of this agreement.
The agreement includes reciprocal access for demersal stocks such as haddock, cod and plaice, which will allow Scottish fishers to catch up to 30,000 tonnes of their existing North Sea quotas in Norwegian waters. Access has also been agreed for North Sea herring in UK waters, and Atlanto-Scandian herring in Norwegian waters, up to a cap of 20,000 tonnes. These are the same levels of access agreed for 2023.
Exchanges of quotas have also been agreed, including an inward transfer to the UK of monkfish, a key stock for many Scottish vessels.
Discussions with the Faroe Islands on further exchanges of opportunities are ongoing.
Cabinet Secretary for Rural Affairs, Land Reform and Islands Mairi Gougeon said:
“Norway is a key partner and we welcome the agreed additional opportunities and flexibility for Scotland’s fishing industry in 2024.
“I would like to thank our negotiators for consistently seeking the best outcomes for Scotland by securing sustainable and evidence-based catching-opportunities.
“This package provides additional opportunities and flexibility for Scotland’s fishing industry and builds on the good relations that we have with Norway, following successful implementation of bilateral arrangements over the last two years.”
Background
The UK signed a Framework Agreement on Fisheries with Norway in 2020. Bilateral exchanges of opportunities were agreed for 2022 and 2023.
Through the UK/Norway agreement, Scottish vessels will be able to fish key whitefish stocks in Norwegian waters of subarea 4 (North Sea), including haddock, cod, monkfish, hake, saithe, whiting, and plaice. Pelagic vessels are granted access to fish Atlanto-Scandian herring.
The UK has exchanged out quotas including Greenland halibut, saithe, ling, and tusk.
The Agreed Record can be viewed here.
The post Bilateral Norway agreement brings additional fishing opportunities appeared first on Marine.
Shaping Scotland’s marine future
We have published our new Marine Science and Innovation Strategy highlighting the crucial role of science and innovation in realising the full potential of the marine environment.
The strategy – part of our Blue Economy Vision to 2045 – is a blueprint for the Scottish Government to utilise the best available science, evidence and data for making informed marine management decisions that benefit the economy, environment and society, including coastal and island communities.
It also includes a commitment for innovation through using the latest technology, such as artificial intelligence, including holographic cameras, drones and submersibles, and non-destructive environmental DNA (eDNA), to model and understand Scotland’s marine environment.
Cabinet Secretary for Rural Affairs, Islands and Land Reform Mairi Gougeon said:
Over a century of science, data and evidence has already shaped our understanding of Scotland’s seas and rivers. Our commitment to science and innovation is not just for exploration but to make a tangible and positive impact for the marine environment, our economy and our cultural heritage.
This ambitious new strategy will give us further evidence to respond to biodiversity loss and the impact of climate change, and to make the most of the opportunities our marine and freshwater environments have for our communities.
The strategy was presented to science, data, analysis and engineering stakeholders at the formal opening of the Helen Ogilvie Hub at the Marine Laboratory in Aberdeen. The hub is named after one of the laboratory’s first female scientists, who was appointed in 1911 and studied plankton in Scottish waters and their role in the marine food chain.
Further information
- One of the first steps will be to map the marine and freshwater science and innovation capability and capacity across Scotland with partners. An operational plan to deliver the strategy will be created, and an Areas of Research Interest (ARI) scoping exercise will enable research priorities and knowledge transfer to key communities of interest.
- A new Scientist-in-Charge (SiC) pathway will increase diversity, inclusion and equality for early career scientists, equipping Scottish Government scientists for leadership roles on land and at sea aboard research vessels.
- The Scottish Government’s Marine Directorate has hundreds of scientists, engineers, analysts and data experts working to deliver impactful research outputs, data modelling which feed into sustainable management of Scotland’s marine and river ecosystems, and science, evidence and data for regulatory and statutory duties, such as monitoring for pollutants in seas.
- The Helen Ogilvie Hub opening event featured a speech from Professor Selina Stead, the UK Government’s Chief Scientific Advisor for the Marine Management Organisation and Executive Dean of Environment at the University of Leeds.
The Marine Science and Innovation Strategy is also available in Gaelic.
The post Shaping Scotland’s marine future appeared first on Marine.